Ways We Turn Biomass Into Energy We Can Use
Abstract
When you ride the autobus to school in the morning, your journey is probably powered by diesel or gasoline, which is both fabricated from petroleum. Petroleum is a fossil fuel , which ways that it is made from decomposed, fossilized organisms – such every bit aboriginal plants, plankton, and algae – that take been buried under the Globe's surface for millions of years.
Fossil fuels like petroleum, natural gas, and coal are taken from deep inside the earth, and used to drive cars, rut buildings, and generate electricity. Petroleum tin also be used to make petroleum-based chemicals (petrochemicals), which are found in many everyday things like the soles of your shoes or the plastic cover of your schoolhouse bus seat.
The good thing well-nigh fossil fuels is that they are very energy dumbo, i.eastward., they contain a lot of energy per unit of measurement of volume. This means that fossil fuels are very good at powering cars and generating heat. The not-and then-good thing well-nigh fossil fuels is that the world has a express amount of them. Considering fossil fuels have millions of years to form, we will eventually use them up earlier more are made. Additionally, called-for fossil fuels or petrochemicals releases the gas carbon dioxide (CO2). COii is known every bit a greenhouse gas, because it can trap the sun's rays inside the globe'southward atmosphere, interim simply like the glass roof on a greenhouse. Called-for fossil fuels raises CO2 concentrations in the atmosphere, and this tin pb to climate disruptions including global warming (1).
Because of these problems, scientists and engineers are working hard to find new kinds of fuels and chemicals that do not add COtwo to the temper, and that can be renewed when supplies run depression. Fuels and chemicals that meet these requirements are referred to as " sustainable ." In an ecology sense, a textile is sustainable if it tin can be used over the long term, without running out or having an overall negative environmental bear on.
Biofuel is one type of fuel that shows a lot of hope for our free energy future, because it is both renewable and environmentally friendly. In other words, biofuel is sustainable.
Biofuels are ordinarily produced from plant materials that cannot exist eaten past humans, such equally corn stalks, grasses, and wood chips. Biomass is another name for the constitute materials that are used to make biofuels. When biomass is harvested and processed, scientists can break downwardly and convert the establish cells into renewable fuels or chemicals. So, instead of waiting a million years for nature to modify plants into fossil fuels, scientists are trying to speed up this process by using clever chemistry to brand biofuel from plants that are alive today.
Now, await a second. If called-for fossil fuels, which are made from ancient organic matter, pumps CO2 into the atmosphere … does not burning biofuels create the same problem? Fortunately, the answer is no. Burning biofuel does indeed release CO2, but recall that the plants used in biofuel are not aboriginal – they were living on the earth at the aforementioned time as you and me. And while nosotros, as humans, breathe oxygen to stay alive, plants instead breathe CO2. This means that because the plants used for biofuel consume CO2 every bit they grow, at that place is no full increase in the amount of CO2 in the atmosphere when they are burned. They are only replacing what they accept taken. In addition, different petroleum, nosotros tin can ever abound new plants for biofuel when we demand them.
Then, if biofuels are sustainable and environmentally friendly, and then they must be the perfect solution to our energy bug, right? Unfortunately, the processes that scientists use to turn biomass into biofuel can be very expensive. Costly chemical reactions mean costly biofuels and bioproducts, and nigh consumers would rather choose regular gasoline or plastic over more than expensive "green" products. In improver, some biofuel reactions crave harsh chemicals that can create their own environmental problems, leaving us right back where nosotros started in terms of sustainability (two).
To see how plants are turned into useful fuels and chemicals, we must get-go understand what they are fabricated of. The walls of constitute cells are responsible for almost all of the weight of a plant and are composed of iii complex molecules called cellulose, hemicellulose, and lignin (Effigy 1).
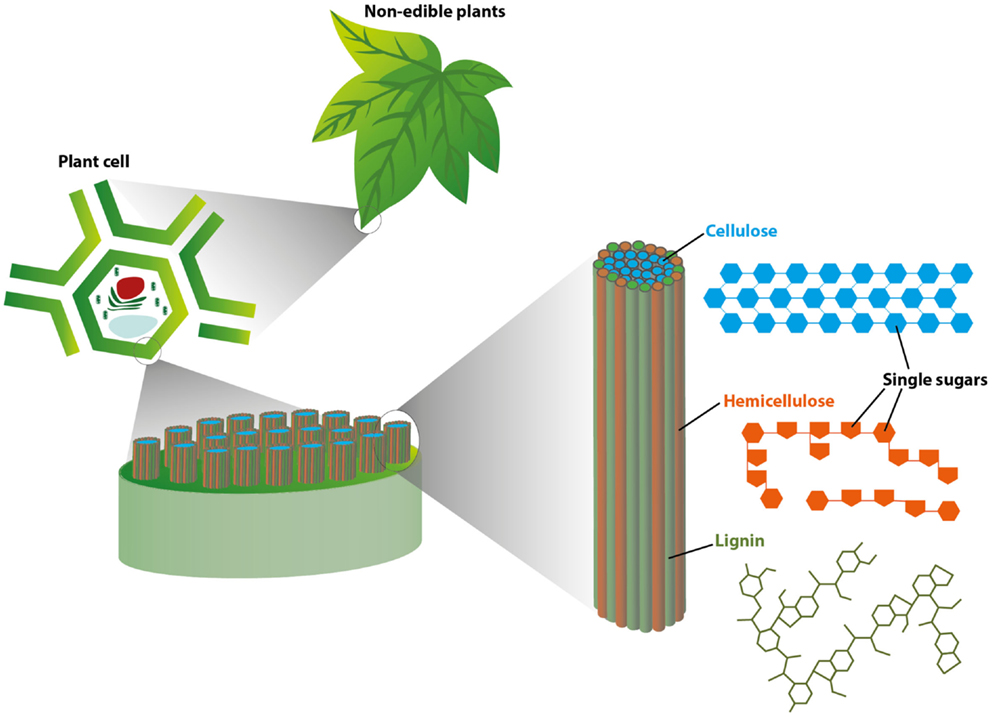
- Figure 1 - This figure illustrates the basic structure of plant tissues, starting at the level of the leaf (Top: "not-edible plants") and zooming in to the cellular level (Left: "plant prison cell").
- As you lot tin run across, at the cellular level, long cellulose molecules (shown in bluish) are tightly packed into bundles surrounded by hemicellulose (orangish) and lignin (light-green). This tightly packed structure helps brand found tissues strong and durable.
The first two molecules, cellulose and hemicellulose, are bursting with unproblematic sugar building blocks, all bound together in a compact structure that is supported past the 3rd molecule – lignin (Figure 1). All three complex molecules in plants must be broken apart to admission the sugar edifice blocks within, which can then be converted to biofuel.
One manner to accomplish this biomass breakdown is to use a lot of harsh chemicals to break autonomously the plant tissues. Withal, these chemicals tin be expensive – even toxic (2). Ideally, nosotros would like to make breaking downward plants easier, and so we do not have to rely equally much on these chemicals.
One possible solution is to use a solvent – a liquid with chemical properties that allow it to dissolve other materials … like plants. Nigh of united states use solvents every day, even if nosotros are not aware of it. For case, yous utilize water equally a solvent every time you wash your hands or brand instant hot chocolate.
Sometimes, only a certain kind of solvent can get the job done. For instance, water may dissolve cocoa powder to make hot chocolate, but information technology would not remove boom smoothen – for that, yous need chemicals called acetone, or ethyl acetate.
Unfortunately, until recently, free energy researchers could not detect a solvent that was (a) cheap, (b) sustainable, and (c) good at breaking down plants. But now, we have discovered a very interesting new solvent chosen γ-valerolactone ( GVL for brusk) that can make biofuel product much cheaper and more efficient (3). GVL is such an interesting solvent because it is not only cheap – information technology is renewable, because it is made from biomass itself.
Nosotros have discovered that nosotros tin can use GVL to extract over seventy% of the original sugars trapped in the dense structure of biomass, to produce simple sugars that are much easier to transform into fuel. This procedure is illustrated in Figure 2, which shows the chemical reaction equally information technology proceeds inside a biofuel reactor. Biofuel reactors are metallic vessels that incorporate biofuel-processing reactions. They are specially designed to withstand the heat, force per unit area, and chemicals involved.
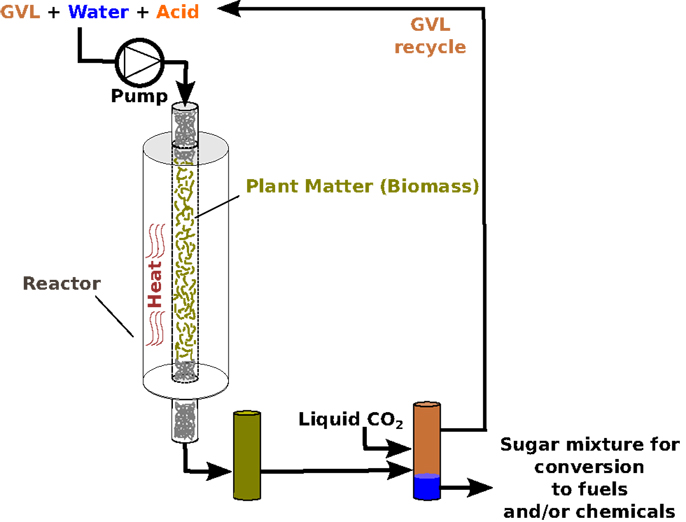
- Figure 2 - Illustration of sugar production from plants, using GVL as a solvent.
Two main backdrop of GVL make it an splendid solvent for saccharide extraction:
(1) GVL gives acids a big heave.
For whatsoever chemical reaction to brainstorm, the ingredients involved (the reactants) must first gather plenty energy. The smallest amount of free energy needed to kick-start a reaction is chosen the "activation energy" (Figure 3). In common biofuel production reactions, lots of acids are mixed with water to assistance suspension downward the biomass. This can take awhile, specially for very tough or woody plants, just calculation GVL to the reaction gives the acids a big energy heave. This boost helps the system gather its activation free energy faster, and then the reaction can go on more quickly (4, 5) (Figure iii).
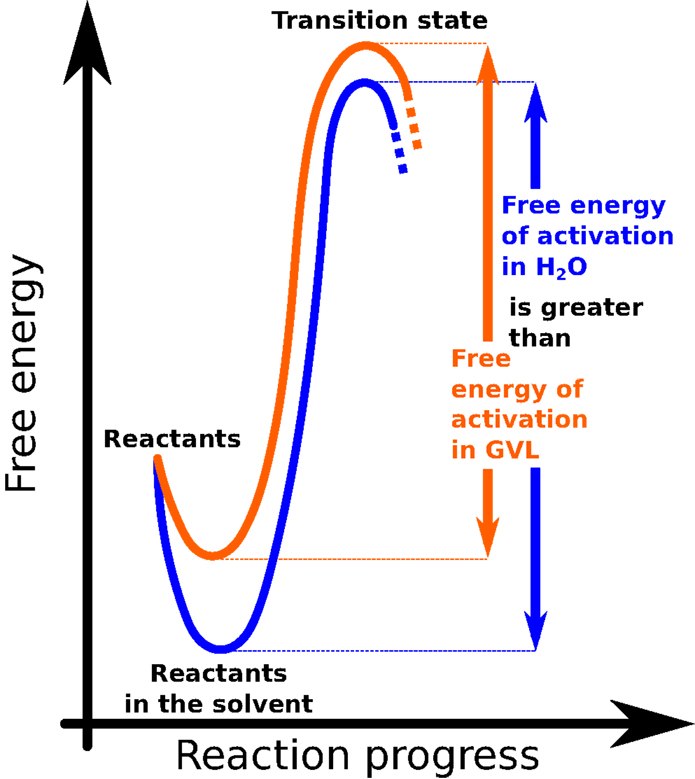
- Figure 3 - This graph illustrates the evolution of a chemical reaction.
- "Complimentary energy" is a fancy mode of referring to the energy that is relevant to the chemical reaction. The "reaction progress" represents the country that the reactants must get through in gild to turn into products.
To illustrate this phenomenon, imagine that ii girls, Gemma and Valerie, are about to race each other to the top of a steep hill. Normally, both runners must stand up behind a starting line to make certain that the race is off-white. Merely in this race, Gemma is really allowed a large head start: when the buzzer goes off, she gets to outset running halfway upwardly the steep loma, while Valerie must begin from the very bottom. Who do you lot remember will win? Y'all guessed it – Gemma gets to the acme of the hill style before Valerie. Simply as the head start puts Gemma closer to the top of the hill in the race analogy, GVL brings the acrid closer to the indicate of reacting with the biomass, allowing the reaction to proceed much faster.
(2) GVL gets lignin out of the way.
To plants, lignin is really important: it gives them their shape and structure, and helps them abound healthy and stiff. But to scientists, lignin is only a nuisance. It is a tough and stubborn molecule that is very hard to break down, and it interferes with obtaining simple sugars from cellulose and hemicellulose molecules. One day, scientist hope to be able to break down lignin itself to make useful things, but for now, they only desire it out of the style. GVL has the unusual ability to dissolve lignin, and to keep it from blocking the big prize: the energy-rich saccharide building blocks.
Possibly, the best thing nearly it GVL that it is can be recycled. At the finish of a biofuel reaction, liquid CO2 can be added to the reactor to split up each reactant into a distinct layer (Figure 2). Think of a bottle of fancy salad dressing: the oil and vinegar, instead of mixing with each other, stay completely separate until the bottle is shaken. Likewise, when COii is added to the biofuel reactor, the GVL and saccharide solution become just similar that salad dressing. The sugars all move into one layer and get concentrated (meet Effigy 2), while the GVL forms its own separate layer. The GVL can then exist easily removed and used once more, while the saccharide solution that scientists end upwardly with is around five times more full-bodied than it would be without GVL. This increased concentration is very important, because it means that you lot demand to spend less energy purifying the concluding production, making the whole procedure more than efficient and less wasteful.
After the GVL has been removed, a concentrated – and very useful – carbohydrate solution is left behind. Scientists accept two options for using this free energy-rich solution:
- They tin upgrade the sugars via further chemical reactions to other useful molecules, which are used to make many items that are derived from petrochemicals today. This means that GVL could be used to produce sustainable alternatives to plastics, soaps, paints, and many other common materials.
- They can "feed" the sugars to microorganisms, such as yeast or leaner, which then metabolize it and produce fuel. The biofuel ethanol is one example: it can power cars and trucks and other machines near as efficiently as gasoline. Some microorganisms have a particularly good appetite for the sugars produced using GVL, because they are gratuitous of the harsh chemicals that are often used in other biofuel reactions. The fact that microorganisms can non but survive but also thrive on GVL-treated sugars means that GVL is suitable for utilise in other biological reactions – not just chemical ones. In this work, microorganisms were used to produce ethanol concentrations so high that it did not cost very much to purify the ethanol into usable fuel.
For all these reasons, using GVL gives scientists promise for creating biofuels and chemicals that can compete with petroleum products in the market place. For centuries now, humans take been inventing new technologies and developing manufacture at an astounding rate – sometimes at a serious cost to the environment. A biofuel production procedure that meets all the requirements of affordability, renewability, and sustainability has the potential to benefit both humans and the earth. With the discovery of GVLs part in biofuel processing, we believe that we are one step closer to a sustainable future.
Glossary
Biofuel: ↑ Certain types of plant matter (see biomass) tin be processed into liquid or gaseous fuels called biofuels. Some biofuels tin can provide renewable alternatives to fossil fuels, such as gasoline.
Biomass: ↑ Biomass is a full general term referring to whatever organic (carbon-containing) cloth that comes from living affair, such equally plants. Constitute biomass is made upwards of 3 main molecules: cellulose, hemicellulose, and lignin. Types of biomass used for biofuels include plants and establish wastes, such as grasses, corn stalks, and forest chips.
Fossil fuel: ↑ Fossil fuels are formed hugger-mugger over millions of years, and are composed of organic matter from the tissues of aboriginal plants and animals. Fossil fuels include coal, natural gas, and petroleum. Petroleum can be refined into other fuels, such every bit diesel and gasoline.
Global warming: ↑ When also much of the gas carbon dioxide (CO2) gets into the atmosphere, it tin trap the sun's rays inside the atmosphere. This phenomenon is chosen the greenhouse effect, and it can pb to an overall increment in global temperatures called global warming.
GVL: ↑ GVL is short for γ-valerolactone. It is a chemic that can be easily made from plants. In our experiment, we used GVL as a solvent to dissolve plants. In the past, GVL has been used in the perfume industry, because information technology has a sweet herbal odor. GVL has also been used in pharmaceutical products.
Reaction: ↑ A chemical reaction occurs when atoms in a substance go rearranged, leading to a chemical change in the substance. A chemical reaction tin can only keep once it has gathered enough energy. This minimum amount of energy needed to outset the reaction is chosen the activation energy.
Solvent: ↑ In chemistry, a solvent is a liquid or a gas that tin dissolve another substance, called the solute. When you lot add together a solvent to a solute, you lot stop up with a solution.
Sustainable: ↑ In an environmental sense, a material is sustainable if it can exist used over the long term, without running out or having an overall negative environmental impact. For example, renewable energy is sustainable considering we tin produce more than of it without causing pregnant damage to the environs. On a larger calibration, an ecological system is sustainable if it can survive over time with healthy levels of biodiversity, productivity, and resources.
Original Source Article
↑ Luterbacher, J. S., Rand, J. M., Alonso, D. Yard., Han, J., Youngquist, J. T., Maravelias, C. T., et al. 2014. Nonenzymatic saccharide production from biomass using biomass-derived γ-valerolactone. Scientific discipline 343:277–280. doi:10.1126/science.1246748
References
[i] ↑ Tester, J. West. 2005. Sustainable Energy. Cambridge, MA: MIT Press.
[2] ↑ Luterbacher, J. Due south., Martin Alonso, D., Dumesic, J. A. 2014. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Dark-green Chem. 16:4816–38. doi: ten.1039/C4GC01160K
[3] ↑ Luterbacher, J. S., Rand, J. K., Alonso, D. M., Han, J., Youngquist, J. T., Maravelias, C. T., et al. 2014. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 343:277–80. doi: 10.1126/science.1246748
[4] ↑ Mellmer, 1000. A., Sener, C., Gallo, J. K. R., Luterbacher, J. S., Alonso, D. M., Dumesic, J. A. 2014. Solvent effects in acrid-catalyzed biomass conversion reactions. Angew Chem. Int. Ed. 53:11872–5. doi: 10.1002/anie.201408359
[five] ↑ Mellmer, M. A., Alonso, D. Yard., Luterbacher, J. S., Gallo, J. M. R., Dumesic, J. A. 2014. Effects of γ-valerolactone in hydrolysis of lignocellulosic biomass to monosaccharides. Green Chem. sixteen:4659–62. doi: 10.1039/C4GC01768D
Source: https://www.frontiersin.org/articles/156397
0 Response to "Ways We Turn Biomass Into Energy We Can Use"
Post a Comment